Introduction
When you first see HCOOCH CH2 H2O, it may look confusing. It is not a standard chemical formula you’ll find in textbooks. However, it seems to refer to the idea of methyl formate + water and possible hydrolysis reaction. In simpler terms, this “formula” may be a shorthand or miswriting of HCOOCH₃ + H₂O (methyl formate reacting with water).
What is methyl formate?
Structure and basic properties
- Methyl formate is an ester of formic acid and methanol. An ester is a compound formed when an acid reacts with an alcohol.
- Its chemical formula is C₂H₄O₂ but more usefully written HCOOCH₃.
- It is a colorless liquid, with a fruity smell. It is volatile (evaporates easily) and flammable.
Because methyl formate is polar and has oxygen atoms, it can interact with water, which opens up the possibility of hydrolysis.
What is hydrolysis of an ester?
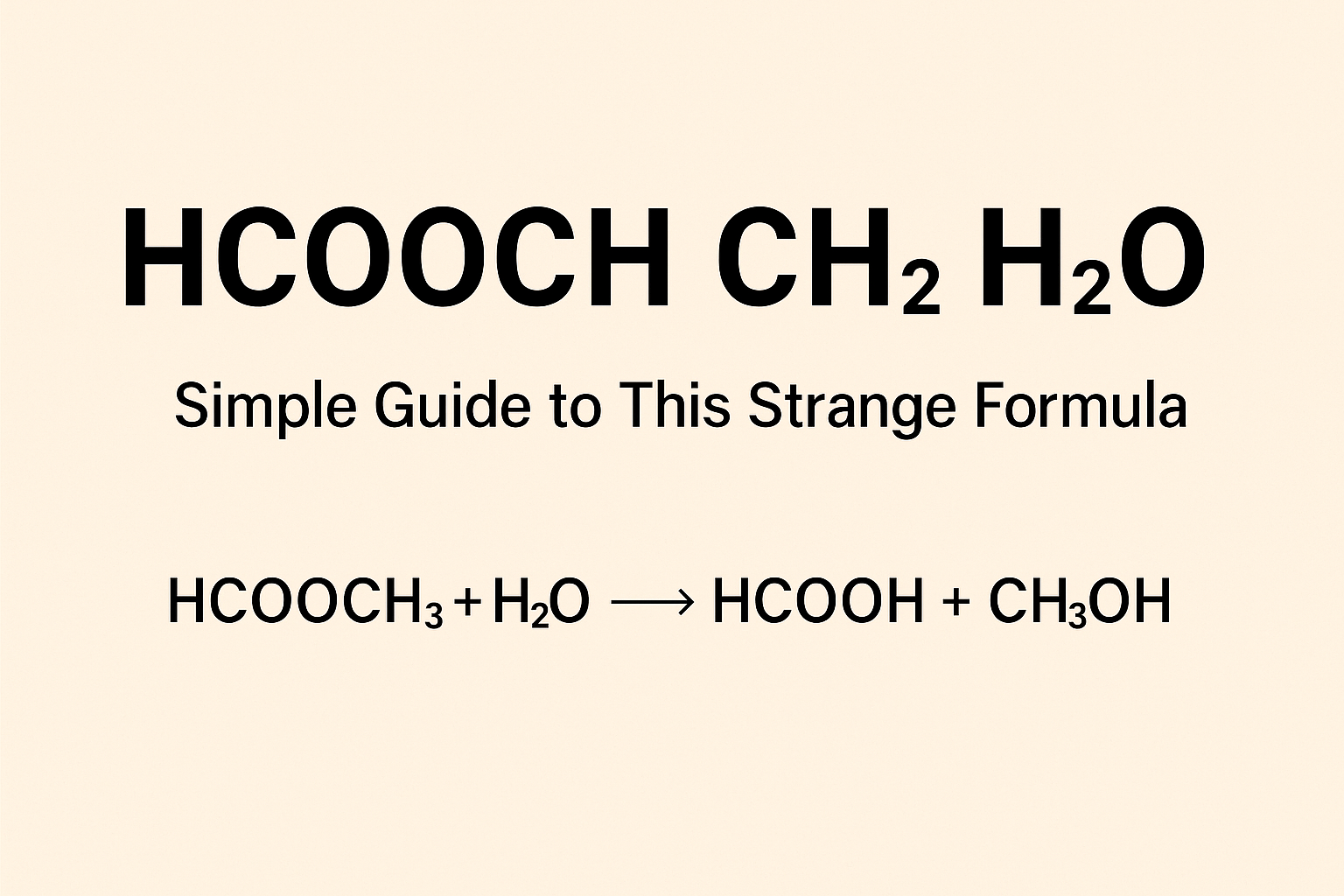
Hydrolysis is a chemical reaction in which water (H₂O) breaks bonds in a molecule. In the case of an ester, water breaks the ester bond, turning the ester into an acid and an alcohol.
For a general ester RCOOR′:
RCOOR′ + H₂O → RCOOH + R′OH
- RCOOR′ is the ester
- RCOOH is a carboxylic acid
- R′OH is an alcohol
In laboratory or industrial settings, this reaction often needs a catalyst (acid or base) to proceed at a useful rate, because without catalyst it is very slow.
Two main pathways:
- Acid catalyzed hydrolysis: uses a dilute acid (like HCl or H₂SO₄) to speed up the reaction.
- Base hydrolysis (saponification): uses a base (like NaOH). This is usually irreversible because the base converts the acid into its salt.
The reaction: methyl formate + water → formic acid + methanol
Putting the specific molecules:
HCOOCH₃ + H₂O → HCOOH + CH₃OH
In words: methyl formate + water produces formic acid + methanol.
This is the key reaction that “HCOOCH CH2 H2O” likely refers to.
Mechanism (step by step, in simple terms)
Under acidic conditions:
- The acid protonates (adds H⁺) to the carbonyl oxygen in the ester, making the carbon more positive (electrophilic).
- A water molecule attacks the carbon (nucleophilic attack), forming a “tetrahedral intermediate” (a temporary structure).
- That intermediate rearranges, releasing the alcohol part (methanol) and forming the acid part (formic acid).
- Finally, a proton is transferred and the products (formic acid and methanol) separate.
Under base conditions, the hydroxide (OH⁻) attacks directly and that leads to a different intermediate, but final products (formate + methanol) arise.
The kinetics (how fast the reaction goes) depend on concentration, temperature, and which catalyst is used. For example, in acid solution, the rate law is:
Rate = k [HCOOCH₃] [H⁺]
This means the rate depends on how much methyl formate is there and the acidity (proton) concentration.
Why this reaction matters
Industrial production of formic acid
One of the main uses is to produce formic acid. In many industrial routes, methyl formate is first made (e.g. from methanol + carbon monoxide) and then hydrolyzed into formic acid.
Because methyl formate can be controlled and separated, this route is practical for large scale formic acid production.
Formic acid has many uses: in leather processing, textile dyeing, preservatives, and more.
Use of methanol
The methanol that results is itself a useful chemical (as a solvent, fuel precursor, or raw material for other chemicals).
Academic and educational importance
This reaction is often used as a model example in organic chemistry courses to teach ester hydrolysis, reaction mechanisms, intermediates, and catalysis principles.
Environmental & green chemistry relevance
Because water is a reactant, and the products are relatively simple and less harmful (if well handled), such reactions are more “green” than processes using harsh reagents.
Also, in soil or wet environments, methyl formate may hydrolyze naturally (though slowly) over time.
Limitations and caveats
- The notation “HCOOCH CH2 H2O” is nonstandard and ambiguous. Chemists prefer precise structural formulas.
- The hydrolysis reaction is reversible (especially under acid). To push it forward, chemists often remove a product or use excess water.
- Safety: methyl formate is flammable, formic acid is corrosive, methanol is toxic. Proper lab procedures are essential.
- Reaction rate: without catalyst or under mild conditions, hydrolysis is slow. That is why catalysts (acid or base) and heating are used.
Interstellar / astrochemistry note
Interestingly, methyl formate (HCOOCH₃) has been detected in space (in interstellar clouds), which makes it one candidate for studying how complex organic molecules form in astrochemistry.
In some studies, formation pathways of methyl formate in icy dust grains include radical reactions (OH radicals, methanol ice etc.).
Thus, the chemistry of methyl formate, water, and related reactions is not only important on Earth but also in understanding chemistry in outer space.
Summary (in simple form)
- HCOOCH CH2 H2O is not a standard formula, but likely means methyl formate + water undergoing hydrolysis.
- Methyl formate (HCOOCH₃) is an ester with useful industrial and chemical roles.
- When methyl formate reacts with water, it yields formic acid (HCOOH) and methanol (CH₃OH).
- The reaction (ester hydrolysis) may need acid or base catalysts to proceed at useful speed.
- This process is key in formic acid production, in chemical education, and has links to environmental and astrochemistry contexts.